
Honeybee Colony Collapse Disorder has always interested me, because I’m interested in insect pathology – and this is probably the most important insect-pathology related event we’ll see in our lifetimes. I’ve written about CCD here at Biofortified, first in my post Colony Collapse Disorder: an Introduction. I followed this up with Are Neonicotinoids the Cause of Colony Collapse Disorder, where I talked about why the pesticide topic was a lot more complicated than neonicotinoid topic alone.
I’ve not been happy with media narratives which focus exclusively on neonicotinoids, because I think the picture is a lot more complicated than one group of pesticides. There are a lot of things which make bees sick, and a lot of these things change the social structure of bees in ways which are negative for the health of the colony. Honeybees also have problems finding food in many areas, which makes these problems worse. So, to restate something I’ve said in previous posts – I don’t think pesticides are entirely blameless, but I think many popular science articles on the topic lay too much blame on pesticides. CCD is multifactorial, with a lot of factors which interact to cause problems.
One question which I’ve had for awhile is: What happens when honeybee colonies Collapse*? In other words, why do the bees leave? A paper in PNAS, Rapid behavioral maturation accelerates failure of stressed honey bee colonies, seems to have answered the question, at least partially.
Continue reading “What makes honeybee colonies Collapse?”
Author: joeballenger2005
Why do we need pest management?
 Everybody needs to eat. Agriculture is the cornerstone of civilization, and by 2100 we’ll need to be a lot better at agriculture because there may be as many as 11 billion people on this planet. Unfortunately, agriculture is also extremely inefficient. For every 100 lbs of food which could potentially be harvested, only about 30 lbs is used by consumers. Some of this is waste, but a lot of this is pest damage.
Everybody needs to eat. Agriculture is the cornerstone of civilization, and by 2100 we’ll need to be a lot better at agriculture because there may be as many as 11 billion people on this planet. Unfortunately, agriculture is also extremely inefficient. For every 100 lbs of food which could potentially be harvested, only about 30 lbs is used by consumers. Some of this is waste, but a lot of this is pest damage.
This is the main challenge for an agricultural scientist: Of 100 lbs of food grown around the world, 70 lbs of it is lost along the way on average. Of those 70 lbs, 35 lbs of that is lost in the field before harvest. If every farmer stopped all pest control measures, that number would increase to 70 lbs of food lost before harvest. Without any additional increases in efficiency between field and table, we would need to increase the amount of land used for agriculture by 30%…or by 136 million acres. Continue reading “Why do we need pest management?”
Help Erika Bueno Fund Her Graduate Project!

Finding funding for some research projects is incredibly difficult, especially for newer researchers. For phenomena which have only been recently described, finding funding is even more difficult than finding funding for more established research areas. Because of this, there are a lot of labs which rely on citizen science projects or volunteer work to gather data. You have a chance to help a really cool and interesting project that can help us understand a new problem that honeybees are facing – through crowdfunding. Continue reading “Help Erika Bueno Fund Her Graduate Project!”
Are Neonicotinoids the Sole Factor Responsible for Colony Collapse Disorder?

A recent paper published in The Bulletin of Insectology claiming that neonicotinoids are the sole cause of CCD has been circulating in the media. The author, Chensheng Lu, has a history of doing research that makes spurious claims about the relationship between CCD and a specific group of pesticides. In this post, I am going to discuss Lu’s research, and use it as a stepping stone to discuss the role that pesticides play in honeybee health.
Why are honeybees exposed to pesticides?
Bees are insects which are raised as livestock, and kept around farms in order to pollinate crops. In order to combat mites which damage adults and spread diseases, beekeepeers use a variety of pesticides. The two most widely used are a pyrethroid called Fluvalinate and an organophosphate called Coumaphos. It is easy to forget that we treat these mites with insecticides, and many popular media reports neglect to mention this completely and instead focus on the agricultural pesticide angle. However, Fluvalinate and Coumaphos are found in virtually all pollen and wax samples. They are frequently found with chlorotalonil, which will synergize the activity of pyrethroids. Coumaphos is the only pesticide found more frequently in non-CCD afflicted colonies. These pesticides are an important part of the honeybee health story. Continue reading “Are Neonicotinoids the Sole Factor Responsible for Colony Collapse Disorder?”
How does the BT protein work?

Life, at its most basic level, is really just a series of chemical reactions. Biochemists and molecular biologists, such as myself, look at how life works at the very most basic level. Unfortunately, this stuff is all very complicated and there are few resources online to explain how this work.
Anastasia has a wonderful post about how Bt corn works in transformed plants, titled simply ‘Bt‘. In the post, she focuses on how the protein works from the angle of a plant biologist. In this post, I will discuss the protein from the angle of an entomologist. Specifically, I will answer this question: What happens when a caterpillar eats the Bt protein?
Contrary to what a lot of people think, Bt is not unique because it’s from an insect pathogen or even because it’s inserted into a plant. Insect pathogens, namely viruses, fungi, bacteria and nematodes, have all been used in pest control operations. Insects which bore into plants are frequently controlled by pesticides which incorporate themselves into plants, like neonicotinoids which are applied as a seed treatment that gets transferred to the plant as it grows. This method isn’t unique to ‘conventional farming’ either, because organic growers will inject Bt into plants to control stem boring caterpillars. Besides, plants have their own insecticides which we consume whenever we eat food. Instead, Bt is unique because the toxins that we use from the bacteria are often only active against very specific groups of insects.
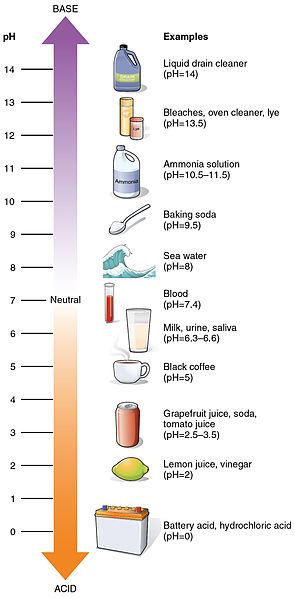
Some Bt proteins work on caterpillars, others work on beetles. All, however work on the gut of the insect. Although I’m not focusing on humans in this post, I would like to compare and contrast the environments of the gut pH between insect and human guts. In humans, the Bt protein is very quickly digested in vitro, and this is due in part to the fact that human and insect stomachs are very different.
Proteins are sensitive to environment, and one very important factor is the pH the protein is in. The pH of human stomach acid is about 2 while the pH of the insect gut is about 10. To give you an idea of how different these environments are, remember that prolonged contact with a highly acidic (pH 2) or a highly basic (pH 10) substance will damage human tissue, which is a pH of about 7. The tissue damage occurs because the local pH is really important for their function. If the pH isn’t within the proper range, the proteins unfold and won’t work. When this happens to human tissue, the result is a chemical burn. Most proteins have a relatively narrow pH window in which they’ll work, and Bt is no different.
Bt toxin requires a high (basic) pH to be active, and must be activated by specific protein-cutting-proteins in the insect gut. The Bt toxin is comprised of a bunch of smaller proteins that work together by teaming up to form holes in the membranes of the cells that form the gut. The holes that are formed are small, and allow salts and other small solutes to get in. When the salts rush in, water follows. When the water flows in, the cells burst. When enough cells burst, the midgut becomes full of large holes. At this point the gut contents spill into the body cavity of the insect, resulting in the death of the insect.
Earlier, I mentioned a wonderful post by Anastasia about the differences between the bacterial proteins and the ones put into corn. However, I do not view the protein encoded by transgenic crops as the toxin. Instead, I view the protein described by Anastasia as part of the toxin. Remember that this gene encodes a protein, and in the gut there are multiple identical copies (subunits) of this protein that hook together to create the pore that kills the caterpillar. This might seem like a pedantic distinction, but it’s very important to understanding how this protein works.
There are two main differences between the proteins found in bacteria and the proteins found in corn. The first is that in the bacterial proteins, there is an extra subunit that has been removed in the genes used in transgenic crops. The second is that one bacteria may produce multiple Bt protein subunits, whereas the corn only produces one subunit*. This is important because those different subunits may interact in different combinations with various potential effects on toxicity and specificity.
So far in this story, the caterpillar has somehow consumed the toxin and if we’re talking about a bacteria derived toxin, the Bt toxin has been activated by the caterpillar’s proteases by cutting off a very specific part from the ‘back’ or, C-terminus of the protein. What we’ve got now is a protein floating in the gut of the caterpillar. In the gut, the protein isn’t going to do a whole lot of damage. To do it’s damage, it has to get down to the cell membrane and latch onto some other proteins and get close to that membrane. Here, we introduce another cast of players:
- Alkaline Phosphatases. Many proteins are turned on and off by the addition of phosphate groups. Under the basic conditions found in the insect midgut, phosphate groups are removed from molecules by alkaline phosphatase. In mammals, the stomach lining is protected by a thick layer of mucus and the pH of this mucus is regulated in part by alkaline phosphatase. In addition, alkaline phosphatase in mammalian intestines have secondary functions which range from regulating the absorption of fat to regulating immune reactions during bacterial food poisoning to regulating interactions between our immune system and beneficial bacteria. Alkaline phosphatases have a lot of functions, many of which aren’t related to digestion.
- Aminopeptidases. Insects consume a variety of proteins in their diets, so digestion involves a lot of proteins that break down other proteins. A lot of these are floating around in the liquid of the guts, but others are attached to the intestinal cells. Some proteins simply cut proteins in random** fashion to speed up digestion by increasing surface area. Aminopeptidases degrade proteins from one end, the ‘front’ or N-terminus, of the protein.
- Cadherins. In mammals, these proteins, along with integrins, play a major role in holding cells together. In insects, they probably play a similar role.
Aminopeptidases, alkaline phosphatases, and cadherins are very common on the gut wall. While the Bt protein is floating in the gut, it eventually runs into aminopeptidases and alkaline phosphatases. Very briefly and very loosely, it binds to these molecules. This binding likely serves to hold the protein to the gut membrane until they run into a cadherin molecule. While the interactions are weak and temporary, eliminating the ability of Bt to bind to these proteins greatly decreases the toxicity of these proteins. The Bt protein eventually finds and binds tightly to a cadherin protein.
While bound to the cadherin protein, the shape of the protein changes in such a way that allows proteases in the gut to cut the protein in a very specific manner. The way this protein gets cut exposes hydrophobic amino acids on the protein. This more or less creates a sticky patch that allows the Bt protein to link up with other Bt protein subunits. After these subunits hook together, they loose their ability to bind to cadherin and then gain the ability to bind very strongly to gut-bound aminopeptidases and alkaline phosphatases. After binding to the aminopeptidases and the alkaline phosphatases, this group of Bt proteins then inserts itself into the cell membrane and forms a pore.
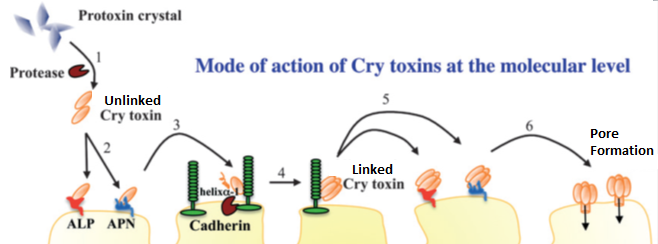
There are still a lot of unknowns about how Bt proteins work, and we’d really like to understand these proteins more so we can engineer new versions. We don’t quite understand what happens between the time the protein leaves the aminopeptidases and alkaline phosphatases and creates a pore in the cell membrane. There’s also some debate on how many subunits form one protein. These might seem like minor things, but even small advances in our understanding of exactly how this protein works goes a really long way in our ability to use these proteins. For example, B. thuringiensis can encode proteins of differing subunits that are toxic by themselves, but when combined together (Cry1Aa and Cry1Ac for gypsy moth larvae) will synergize each other and become more toxic than either protein alone. Eliminating certain parts of the protein may also change toxicity. If we could figure out how to do this in a predictable fashion, we could increase the number of proteins created by transgenic plants while simultaneously minimizing the number of genes we need to insert into the plant. It would also be possible to predict how insects will become resistant to Bt, and which modifications of the protein would allow us to overcome this resistance. This, in turn, would likely make the evolution of Bt pesticide resistance far more difficult for crop pests.
So there you have it…this is how Bt works. It’s a very complicated process, but when you understand some of the biochemical details it’s actually a lot less complicated than it would initially seem. We don’t know everything about how the protein works, but we do know enough to be able to use it. As we find out more about the protein, and figure out new ways to manipulate it, we can hone and sharpen this tool so that it will be useful for years to come.
*Stacked corn produces two different subunits which don’t bind together.
**Not exactly random – cuts are made after specific amino acids. This just serves to break the proteins into smaller chunks to increase the rate of digestion. So, for our intents and purposes it’s all random. It’s not really, but these are pedantic differences that don’t matter for this discussion.
Works Cited
- Harris T.J.C. & Tepass U. (2010). Adherens junctions: from molecules to morphogenesis, Nature Reviews Molecular Cell Biology, 11 (7) 502-514. DOI: 10.1038/nrm2927
- Lallès J.P. (2010). Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet, Nutrition Reviews, 68 (6) 323-332. DOI: 10.1111/j.1753-4887.2010.00292.x
- Pardo-López L., Soberón M. & Bravo A. (2013). Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection, FEMS Microbiology Reviews, 37 (1) 3-22. DOI: 10.1111/j.1574-6976.2012.00341.x
Strepsipteran genome brings us a step closer to solving an entomological enigma

Parasitoids play a huge role in regulating insect populations. They have a lot of tools to do this, including using genetic modification to disable the immune systems of their hosts, as I described in Polydnaviruses: Nature’s GMOs. In agriculture, if you remove parasitoids, you end up applying far more insecticides… and nobody wants that. In apiculture, removing parasitoids such as Apocephalus borealis could help honey bee populations. Parasitoids are important.
The Strepsiptera are one group of Parasitoids that I haven’t yet discussed. These are particularly bizarre insects. The males are relatively unremarkable… they’ve got six legs, antennae and wings. Relatively bug-like. The females, however, don’t even resemble insects. Out of all the Strepsiptera, the genus Xenos has been best described (shown here). The bottom picture is a male. The top picture, however, is a female. Yeah… they’re the same bug.
This is where things get freaky. In more ways than one. Continue reading “Strepsipteran genome brings us a step closer to solving an entomological enigma”
Colony Collapse Disorder: An Introduction
Shortly after I graduated high school, commercial apiaries started to report massive losses of honeybees. Honeybees are probably the most economically valuable insects in the world, and are responsible for pollinating most of the food we eat. Here in the United States there’s an entire industry built up behind honeybees, with most US honeybees being transported to California to pollinate almonds at some point in the year.
 Unfortunately there are a lot of wrong-headed things out there in the press. One common idea I see spread through facebook meme, such as the image to the left, is that biotech crops are responsible for killing the bees. This is a hypothesis that’s been pretty thoroughly researched in a variety of ways. Industry data very strongly indicates this is not the case: a recent meta analysis performed by Monsanto published in PLOS ONE reviewed experiments done by a wide variety of researchers and concluded that there were no effects on survival of bees on Bt crops. Academic research is consistent with the industry data, from a 2005 review on the nontarget effects of Bt crops in the Annual Review of Entomology:
Unfortunately there are a lot of wrong-headed things out there in the press. One common idea I see spread through facebook meme, such as the image to the left, is that biotech crops are responsible for killing the bees. This is a hypothesis that’s been pretty thoroughly researched in a variety of ways. Industry data very strongly indicates this is not the case: a recent meta analysis performed by Monsanto published in PLOS ONE reviewed experiments done by a wide variety of researchers and concluded that there were no effects on survival of bees on Bt crops. Academic research is consistent with the industry data, from a 2005 review on the nontarget effects of Bt crops in the Annual Review of Entomology:
Neither Bt cotton nor Bt maize requires bees for pollination, but cotton nectar is attractive to them and produces a useful honey. Maize pollen may be collected when other pollen sources are scarce. Pre-release honey bee biosafety tests have been conducted for each Bt crop registered in the United States, including Cry9C maize and Cry3A potatoes. Each test involved feeding bee larvae and sometimes adults with purified Cry proteins in sucrose solutions at concentrations that greatly exceeded those recorded from the pollen or nectar of the GM plants in question. In each case, no effects were observed. The rationale for requiring larval and not adult bee tests is questionable, because adult bees ingest considerable quantities of pollen in their first few days post emergence. Larvae, particularly later instars, also consume pollen along with jelly secreted by nurse adult bees, but only recently have there been attempts to quantify pollen ingestion by individual larvae. Other studies with bees fed purified Bt proteins, or pollen from Bt plants, or bees allowed to forage on Bt plants in the field have confirmed the lack of effects noted by the U.S. Environmental Protection Agency (EPA). Post-release monitoring programs are now underway to assess impacts of North American GM crops on pollinators under commercial field conditions.
Admittedly, there could be better data on this subject. For instance some of the research that’s been done has been done without some essential controls, like this German group which fed honeybee larvae a mixture of Bt pollen without determining that active Bt proteins were present in the pollen. The data, however, is generally against the idea that Bt crops harm bees. Continue reading “Colony Collapse Disorder: An Introduction”
Get involved in citizen science… help the honeybees!
While researchers have been making progress in discovering causes of Colony Collapse Disorder (CCD), we still don’t have all the answers.
Some of you may remember awhile back when I wrote about a fly called Apocephalus borealis. This fly was discovered by San Francisco State University researchers to be parasitizing honeybees.
There are a lot of questions that need to be answered before we can even think about associating the fly with CCD, and the authors of the original paper are now looking to get the public involved to help answer a lot of these questions.
Continue reading “Get involved in citizen science… help the honeybees!”
No, spiders are not invading India.
When it comes to public relations, few animals have problems as severe as spiders. There are two kinds of confirmed medically significant species of spider in the US, the widows (genus Latrodectus) and the recluses (genus Loxoceles). Although diagnoses of widow bites are probably fairly accurate, there is a huge problem with the overdiagnosis of recluse bites. In fact it’s very common for brown recluse bites to be diagnosed in areas where they have never been found and those who live in houses infested with brown recluses generally don’t get bitten. In fact, if someone claims to have been bitten by a brown recluse I generally assume they’re mistaken unless they actually captured the spider and live in the area where brown recluses are found.
Furthermore, there are entire species which are thought to be medically significant without evidence they cause harm in humans. The hobo spider, Tegeneria argestis is a prime example of such a species. The spider is widely reported to be medically significant, yet the only human evidence for this comes from highly dubious case reports where there is either no evidence the spiders bit the people in question or where the spider was not collected from the site of the bite. In fact, a recent article in the Journal of Medical Entomology indicates the venom of this spider cannot break red blood cells (which are relatively weak), and that they are not able to transport bacteria like MRSA which could cause skin lesions. In short, relying on eyewitness identification of spiders without collected specimens can result in quite a few mistaken diagnosis when it comes to spider bites.
So imagine my surprise when I stumbled across this article from the Daily Mail claiming that spiders are rampaging through the town:
And scores more have been treated in hospital after the town was suddenly invaded by the poisonous eight-legged creatures last month, which have left residents living in a state of panic.
Continue reading “No, spiders are not invading India.”
The world’s smallest fly is a Phorid!
Longtime readers might know I have a bit of an affinity for a specific family of flies, the Phorid flies. I think they’re cool because many have either decomposer or parasitoid habits. The last time they were in the news, it was because a North American species had been discovered to be parasitizing honeybees in California. However, they ended up in the news again while I was traveling in Ecuador. Frank and I are working on a 5 part article on our travels, but first let’s discuss ant-decapitating flies.
I briefly discussed why I think phorids are so neat in Apocephalus borealis, a new threat to honeybees? but wanted to touch on it again. Many of these guys are decomposers. One very common member of this group, the Megaselia coffin flies, will lay their eggs on anything stinky and are often found around neglected trashcans and are sometimes confused with fruit flies. Like any large insect family, there’s a diversity of life cycles and not every species fits this mold. My last phorid-centered article focused on the genus Apocephalus, whose larvae feed inside the body cavities of honeybees. Some phorids, however, have much more specific niches. Continue reading “The world’s smallest fly is a Phorid!”








