By Alma Laney and Alison Bernstein
This post is the second in a series about transgenerational inheritance, epigenetics, and glyphosate that address questions raised by the publication of the paper, Assessment of Glyphosate Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutations: Generational Toxicology.
Guidelines for studying epigenetic inheritance
A 2017 paper mentioned, “A guide to designing germline-dependent epigenetic inheritance experiments in mammals”, provides guidelines for properly designing studies of germline epigenetic inheritance to control for the variables that we touched on in the above section. We will summarize the main points here and compare the recommendations to the design of the study under discussion.
Animal strain
Prior studies (including from the lab that published this glyphosate paper) suggest that outbred strains of animals are more sensitive to transgenerational impact of toxicant exposures and diet, and that different strains of mice show different susceptibility to these effects. Outbred mice are more similar to a human population than inbred mice. However, experiments with outbred strains become very difficult to interpret because every mouse is genetically different, undermining the purpose of these experiments to isolate a transgenerational epigenetic effect from a genetic effect.The guide to epigenetic inheritance experiments states: “unless there are clear reasons to choose an outbred strain, we recommend using inbred strains to remove genetic variability and aid the interpretation of epigenetic data.”
The glyphosate paper used an outbred strain, which provides a huge confounding effect to their results. The genetic differences they identified after 3 generations of breeding are not at all accounted for. In fact, their own data suggests that genetics plays a huge role (see section on founder effects) that is not adequately addressed.
The choice of an outbred strain for a transgenerational study creates many issues for interpretation of results. However, much has been said in online discussion about this paper about the specific choice of Sprague Dawley rats for this study so it is worth exploring if this is a concern. These rats are prone to spontaneous tumors, with most tumors occurring after one year of age. This 1992 paper explores rates of spontaneous neoplastic lesions of Sprague Dawley rats that were used as controls in 17 chronic toxicity/carcinogenicity studies from 1986-1992. Of 1340 male and 1329 female rats, ~15% of males and ~22% of females died or were sacrificed due to the presence of neoplasms by 2 years of age and none of these appeared until 15 months of age. The paper also characterizes non-cancerous health issues in these animals.
These age-related spontaneous tumors are a particular concern in any study where rats are aged. However, in this study, animals are euthanized at 12 months so it is not a major concern, but tumor incidence, location, and type should be tracked. Control animals would be the baseline comparison to look for an increase over the base rate of cancer incidence.
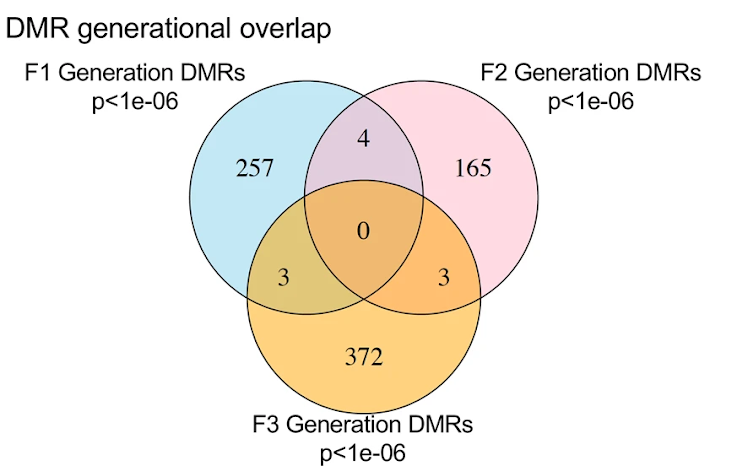
In the current paper, they lumped together all “disease,” despite what appears to be an in-depth pathological analysis. For example, in the testes they looked at markers of cancer and infertility but lumped them all into a binary classification of disease/no disease. In other tissues, the identified both cancer-related and non-cancer related pathologies, but also lumped these into the same binary classification scheme. It seems odd to reduce such an in-depth pathological analysis to a binary disease/no disease variable. Doing so skews the statistics in favor of obtaining a statistically significant result. The 1992 paper cited above that described the spontaneous neoplasms in aged Sprague Dawley rats provides a contrast for how the authors separated out various types of cancer by tissue. The choice to lump these all together makes interpretation of these results difficult, especially given the choice of strain.
Matrilineal vs patrilineal breeding
Because these studies span multiple generations of animals, well designed breeding schemes are critical and reporting of these breeding schemes in the manuscript is equally important. Because environmentally induced changes can be inherited from mother, father or both parents, it is important to control for these in an experiment.
For this reason, the authors of the review recommend not using a dual breeding group in which exposed males and exposed females are bred together.
Breeding designs that assess these options require exposing females and males of the parent generation to the environmental factor under study (e.g., experimental diet and control diet), then breeding the exposed females to control naive males (matriline) and exposed males to control naive females (patriline). A ‘dual breeding group’ in which exposed males are bred with exposed females can also be included to test for possible interacting effects that may be different than effects resulting from transmission through only one parent, but dual breeding should always be complemented by matrilineal and/or patrilineal breeding.
This is not what was done in the glyphosate paper. Males and females were exposed and crossbred at each generation without complimentary matrilineal or patrilineal breeding. The authors explain the breeding strategy in the results section.
No sibling or cousin breeding (crosses) was used in order to avoid any inbreeding artifacts in either the control or glyphosate lineages. Generally, 6–8 founder gestating females from different litters were bred, and 5 animals of each sex from each litter used to generate 25–50 individuals of each sex for each generation for analysis, as previously described.
This indicates that only a dual breeding strategy was employed at all generations, such that the lineages are now intertwined and not independent (which will be important for the discussion of statistics later). While this outbreeding does counteract the effects of using an outbred strain discussed above, it does create a different set of concerns.
With multi-generational studies such as this one, it is difficult to design a breeding strategy to keep these completely independent without needing to generate a very large number of animals. Thus, careful reporting and proper analysis become even more critical. This example of a well reported study allows the reader to understand and track each generation back to the original generation, explains that this was matrilineal and details how they chose mating pairs at each generation.
Duration of cohabitation during mating
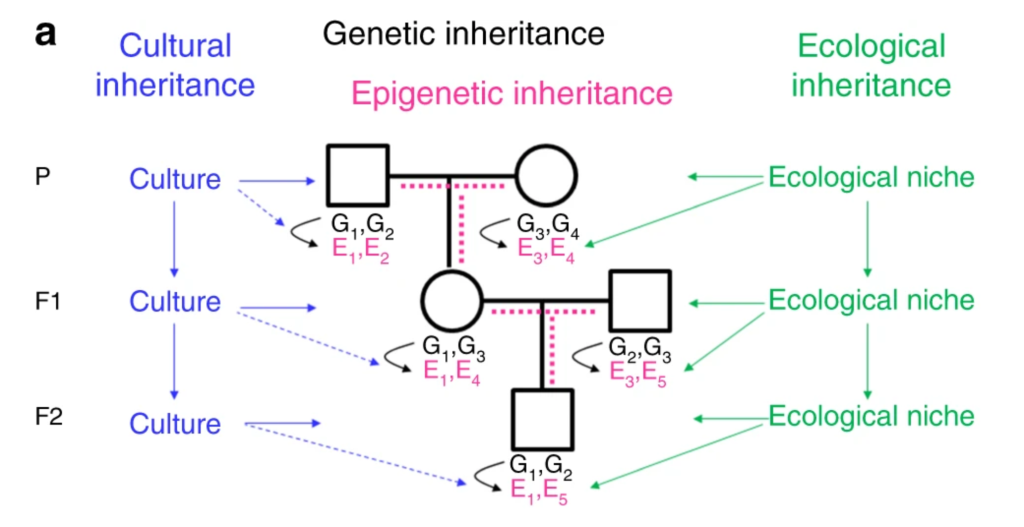
As mentioned above transgenerational inheritance of traits can be mediated by epigenetic, ecological, or cultural mechanisms. In matrilineal experiments, it is critical to design experiments to allow the separation of germline effects from prenatal or postnatal variables that are known to have lifelong effects on offspring. Many researchers choose patrilineal breeding schemes since male rodents can be separated from the female after mating. However, if a treatment alters the health, appearance or behavior of males, this may affect females during mating and cause changes in offspring in ways independent of germline transmission.
There are multiple ways to deal with this and the considerations for each of these experimental design choices are discussed in detail in the aforementioned guide. However, even when choosing one of these proposed methods to control for these effects, it is still possible for males to impact females during cohabitation. Thus the only way to definitely rule out these other effects is to use assistive reproductive technology, a topic which is also discussed in the guide. The glyphosate paper provides no information regarding duration of cohabitation during mating.
Group size
One of the most important steps in experimental design is the determination of group size for statistical analysis. Group size is typically estimated prior to the start of an experiment based on the expected effect size, expected variability of the effect based on pilot studies or published data, and the expected distribution of data. Then, researchers work backwards to dose the appropriate number of animals in the parental generation. This paper does not indicate how many male and female animals were exposed in the parental generation. In addition, the group size varies between control and glyphosate and from generation to generation without any justification provided for why these sizes were set and why they are so different between.
A related consideration is to determine ahead of time the statistical test that will be done to ensure that the selected group size is adequate to detect a difference. Inadequate sample size planning can doom a study before it starts, creating ethical and resource issues. A sample size that is too small will miss real associations and find associations that don’t exist. This would be an ethical concern because an experiment that cannot possibly answer the question at hand is a waste of animals and inconsistent with the 3 Rs of animal research (Replacement, Reduction and Refinement). It is also a waste of time and resources. A sample size that is too large is also an ethical concern due to the unnecessary use of animals that aren’t needed to answer the questions at hand and uses unnecessary resources.
Selecting animals for breeding
This is a critical point that often goes unmentioned in methods and this study is no exception. From each litter, at each generation, there is a range of phenotypes observed in almost every experiment. Thus, researchers are faced with a choice of which animals from each litter to choose for various analyses and for breeding the next generation. There are multiple options available. If tests can be done prior to sacrifice, animals can be tested prior to breeding to help choose breeders, animals can be tested after breeding, or different animals can be used for analysis and breeding. Tests that are done after sacrifice can be done after breeding to acquire data on the animals that contribute to the lineage or separate animals can be used for analysis and breeding. Another possibility is to assign animals at random for breeding and other endpoints. Each of these scenarios has pros and cons. These issues is discussed in detail in the guide.
It is unclear from the methods how the authors selected animals for behavior tests, postmortem analysis, sperm collection and further breeding. The additional variation and founder effects introduced by choosing an outbred strain makes the need for appropriate choices here even greater. Again, this lack of detail in the methods combined with the choice to use an outbred strain and the observed founder effects, makes it very difficult to interpret the reported results. At a minimum, these decisions need to be reported.
Avoiding litter and cage effects
It is a well-known phenomenon that animals from the same cage or litter are more similar on behavioral and molecular measures than animals from different cages or litters. Studies must be carefully designed in regards to weaning strategies and these choices must be reported. Weaning is when pups are separated from the mother, split by gender and assigned to new cages. The main decision to make at the time of weaning is whether same-sex siblings of a given litter should be housed together or cross-fostered with same-sex animals from other litters.
An important recommendation in the guide to preventing litter effect is to keep the number of animals per cage constant, or at least within a narrow range, within and between experimental groups, especially for behavioral tests where the number of animals per cage is known to impact behavior. There are multiple strategies available (culling or cross-fostering, for example, summarized in the guide) and researchers must make choices about how to design their experiment to best control for these issues. If it is not possible to choose animals from independent litter, litter must be included in the statistical model.
As with many of the other experimental design options discussed, each choice has advantages and disadvantages. This underscores the need for reporting the weaning strategy in the methods section. There is also a known effect of number of pups during gestation on a variety of phenotypes. Some studies get around this by only using litters of a given size for subsequent stages. No information regarding weaning strategies or litter size is provided in the glyphosate paper.
Defining the experimental unit
This is related to both avoiding litter and cage effects, establishing group size and choosing the appropriate statistical test. As described in the guide, the experimental unit is the entity within an experiment that is assigned to a group and is the unit of statistical analysis. For a patrilineal breeding scheme, each individual male mouse is an experimental unit. For a matrilineal breeding scheme, the exposed female mouse is the experimental unit. All pups from a litter are technically part of the same experimental unit and are considered as one sample.
This also highlights some of the experimental design issues with a dual breeding schemes that we discussed earlier. Littermates cannot be considered as independent measures. Failure to appropriately consider that mothers are the experiment unit and using individual pups as the experimental unit causes inflated false positive rate (Type I errors). This applies at each generation, which largely drives the need for very large numbers of animals for transgenerational studies. The authors of the transgenerational methods guide recommend the following:
This issue needs to be addressed during experimental planning by using power analyses to estimate the number of litters required for the experiment, counting the number of litters in each generation as the experimental unit. Then, animals of different litters can be assigned to different behavioral or molecular tests and for breeding. Alternatively, multiple animals from the same litter can be tested, but their scores should either be averaged and considered as a single sample, or researchers can employ a mixed-effects model of analysis, which ensures that animals are nested within litters.
Very little information is provided in the methods of the glyphosate paper regarding these issues. There is no information provided about litter mates; the inconsistent number of animals used per group suggests that some of these animals were actually littermates. Relying on the numbers as reported and the data provided in the supplement raises major concerns about whether the researchers properly defined the experimental unit, adequately planned group sizes or controlled from litter and cage effects.
In conclusion, the study design as described is inadequate to answer the question of whether glyphosate exposure induces transgenerational epigenetic inheritance in the F3 generation. To give the authors the benefit of the doubt, this may be a problem of reporting and the study design may be adequate. Even going back to previous papers from this lab looking at transgenerational epigenetic inheritance with other toxicants did not add clarity on many of these issues. Many of these studies suffer from some or all of these same issues.
This lab has developed their system and are using their system to test a variety of different compounds. There is nothing necessarily wrong with this “plug and play” approach, if the methods utilized are appropriate and the system has been demonstrated to work to answer the questions at hand. Given the possible methodological problems here and in other papers, this “plug and play” approach does not seem appropriate here. They appear to be using the same design over the course of years without critically assessing if this design works as the field learns and progresses.
The purpose of a scientific paper is to provide adequate information to the reader to understand and interpret the results. Because of the poorly reported methodology, the paper as written raises so many questions that the results of this study are largely uninterpretable. Even if there were nothing wrong with the data collection or statistics, it would be extremely difficult to draw any conclusions from the information provided because of the methodology questions. The methods, as reported, are inadequate to determine if transgenerational epigenetic inheritance is occuring.
Given these experimental design problems, we could potentially dismiss this paper without getting into the details. However, it is a useful exercise to explore the other issues that further complicate and confound the reported results, even if we assume that all of these issues with methods can be explained by poor reporting and that the study design is actually ok.
View the other parts of our series on transgenerational epigenetic inheritance: